Summary
- Profile Type
- Technology offer
- POD Reference
- TOES20250709023
- Term of Validity
- 9 July 2025 - 9 July 2026
- Company's Country
- Spain
- Type of partnership
- Research and development cooperation agreement
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
-
A private Spanish company specialized in hardware development (innovative solutions) offers a compact, oil-free freeze-drying system for autonomous stabilization of biological samples.
Based on solid-state refrigeration and oil-free vacuum technology, it ensures safe and precise lyophilization without compressors or CFCs (Chlorofluorocarbons).
Ideal for biobanks, clinical logistics, and space biology. R&D collaborators are sought to deploy, adapt, and integrate the system in real-world enviro - Full Description
-
1. This freeze-drying system has been jointly developed by two Spanish SMEs: one specialized in technological solutions for life sciences and another in advanced hardware design. Together, they have created the freeze-drying system designed to provide a compact, autonomous solution for preserving biological samples under controlled vacuum and temperature conditions, without relying on conventional mechanical refrigeration or oil-based components.
2. The technology addresses the critical need for safe, autonomous stabilization of biological samples, particularly in settings where conventional refrigeration systems are impractical due to size, maintenance requirements, or environmental constraints. Traditional lyophilization equipment relies on compressors, oil-based pumps, and refrigerants—factors that increase energy consumption, limit portability, and raise contamination risks. These challenges are especially significant in remote medical logistics, space biology, and decentralized biobanking infrastructures.
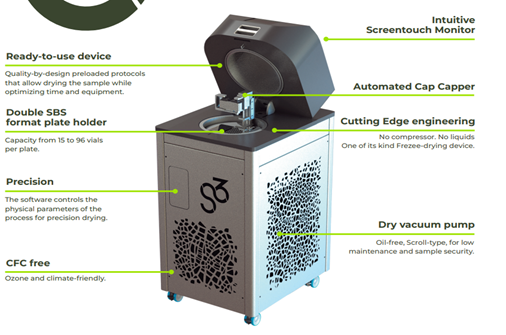
3. The proposed system is a compact, oil-free freeze-dryer that integrates solid-state cooling and a dry scroll vacuum pump to create and maintain deep vacuum conditions (≤ 0.05 mbar) without the use of CFCs, oil, or moving parts. It includes a programmable touchscreen with preloaded drying cycles, a dual SBS-format sample shelf (15–96 vials per tray), and a mechanical stoppering system that seals vials after dehydration.
4. The main innovation lies in its compressor-free, solid-state architecture and oil-free vacuum generation, which eliminate the most failure-prone elements of conventional systems.
5. The SME´s seeks research and development cooperation agreements with partners interested in adapting and integrating this technology into new application scenarios, validating its performance in diverse or extreme operational environments, or co-developing enhanced features aligned with specific technological or scientific requirements.
TECHNICAL SPECIFICATION OR EXPERTISE SOUGHT
The lyophilization system is a fully developed, commercially available technology based on solid-state refrigeration and oil-free vacuum generation. It achieves deep vacuum levels of ≤ 0.05 mbar and operates across a thermal range of −65°C to +70°C. It has already proven effective for standard laboratory samples.
The objective is to identify technical partners capable of exploring new application scenarios for the system beyond conventional laboratory or biobanking use. These include, but are not limited to:
• Integration into mobile health platforms, environmental diagnostics, or resource-limited infrastructures.
• Use with non-standard sample carriers
• Deployment in workflows requiring minimal maintenance, silent operation, or cleanroom compliance.
The technology owner seeks partners with specific expertise in:
• Low-pressure instrumentation and vacuum monitoring under field conditions.
• Preservation of thermolabile or moisture-sensitive biological materials (phages, RNA, probiotics).
• Development and validation of freeze-drying protocols for unconventional biomatrices.
• Hardware-software integration (API control, system interfacing) in automated sample preparation chains.
The aim is not to modify the core technology (unless necessary), but to technically validate and adapt it to new operating contexts and biological formats. - Advantages and Innovations
-
This freeze-drying system introduces significant innovations in design, operation, and applicability when compared to conventional lyophilizers. Its core differentiator is the elimination of compressors, oil-based components, and refrigerants, achieved through a fully solid-state cooling platform and an oil-free scroll vacuum pump. This architectural shift results in a more compact, reliable, and low-maintenance device that is uniquely suited for autonomous or mobile environments.
Unlike standard systems, this unit operates silently (<62 dB) and with minimal energy consumption, enabling its deployment in noise-sensitive or low-resource settings. Its CFC-free design aligns with climate resilience goals and eliminates the need for hazardous substances. The solid-state architecture also removes most moving parts, drastically reducing mechanical wear and service needs over the product’s lifecycle.
Precision is another innovation: the system maintains tight control of thermal cycles (−65°C to +70°C) and vacuum levels (≤ 0.05 mbar), which are critical for the successful stabilization of biological samples. A mechanical stoppering system allows for hermetic sealing of vials after dehydration, preserving sample integrity during storage and transport.
The unit is fully enclosed and certified for safe handling (IP20), including a programmable touchscreen interface with validated protocols, dual SBS-format trays for flexible vial accommodation, and an integrated defrosting system with fluid-level monitoring. These features make it easy to operate while offering high technical performance in a compact footprint.
Together, these innovations position the system as a next-generation solution for biobanking, diagnostic sample logistics, and space biology—bridging the gap between laboratory-grade lyophilization and autonomous, field-deployable systems. - Stage of Development
- Already on the market
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- Goal 13: Climate Action
- Goal 9: Industry, Innovation and Infrastructure
- Goal 12: Responsible Consumption and Production
- Goal 11: Sustainable Cities and Communities
- IPR status
- IPR granted
Partner Sought
- Expected Role of a Partner
-
TYPE OF PARTNER:
The technology provider seeks collaborations with universities, applied research institutes, diagnostics manufacturers, and biomedical companies. Relevant fields include biotechnology, clinical diagnostics, bioanalytics, or mobile healthcare solutions.
ROLE OF THE PARTNER:
• Define target use-case: The partner will propose a realistic deployment scenario (decentralized clinical testing, field sampling, remote biobanking) where freeze-drying is critical for sample preservation.
• Pilot the technology in that context: The partner will implement and test the lyophilizer under relevant environmental, biological, and operational conditions, generating technical data on performance (achieved vacuum, cycle duration, temperature stability, device autonomy).
• Contribute application-specific requirements: Identify necessary adjustments to trays, formats, vial interfaces, or dehydration protocols for sample types outside conventional blood/plasma workflows (e.g. PCR reagents, saliva filters, insect material).
• Participate in integration efforts: For partners with system-level capacity, help define hardware or software interfaces to embed the lyophilizer into broader automated or mobile workflows (interfacing with extraction or analysis modules). - Type and Size of Partner
- Other
- R&D Institution
- SME 11-49
- SME 50 - 249
- University
- Big company
- Type of partnership
- Research and development cooperation agreement
Dissemination
- Technology keywords
- 06001005 - Diagnostics, Diagnosis
- 06002006 - Synthetic Biology
- 06001002 - Clinical Research, Trials
- 06001013 - Medical Technology / Biomedical Engineering
- 05001001 - Analytical Chemistry
- Market keywords
- 05001001 - Diagnostic services
- 05001007 - Other diagnostic
- 05005022 - Other clinical medicine
- 05004004 - Medical instruments
- Targeted countries
- All countries