Summary
- Profile Type
- Technology offer
- POD Reference
- TOIT20240724004
- Term of Validity
- 24 July 2024 - 24 July 2026
- Company's Country
- Italy
- Type of partnership
- Investment agreement
- Commercial agreement with technical assistance
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
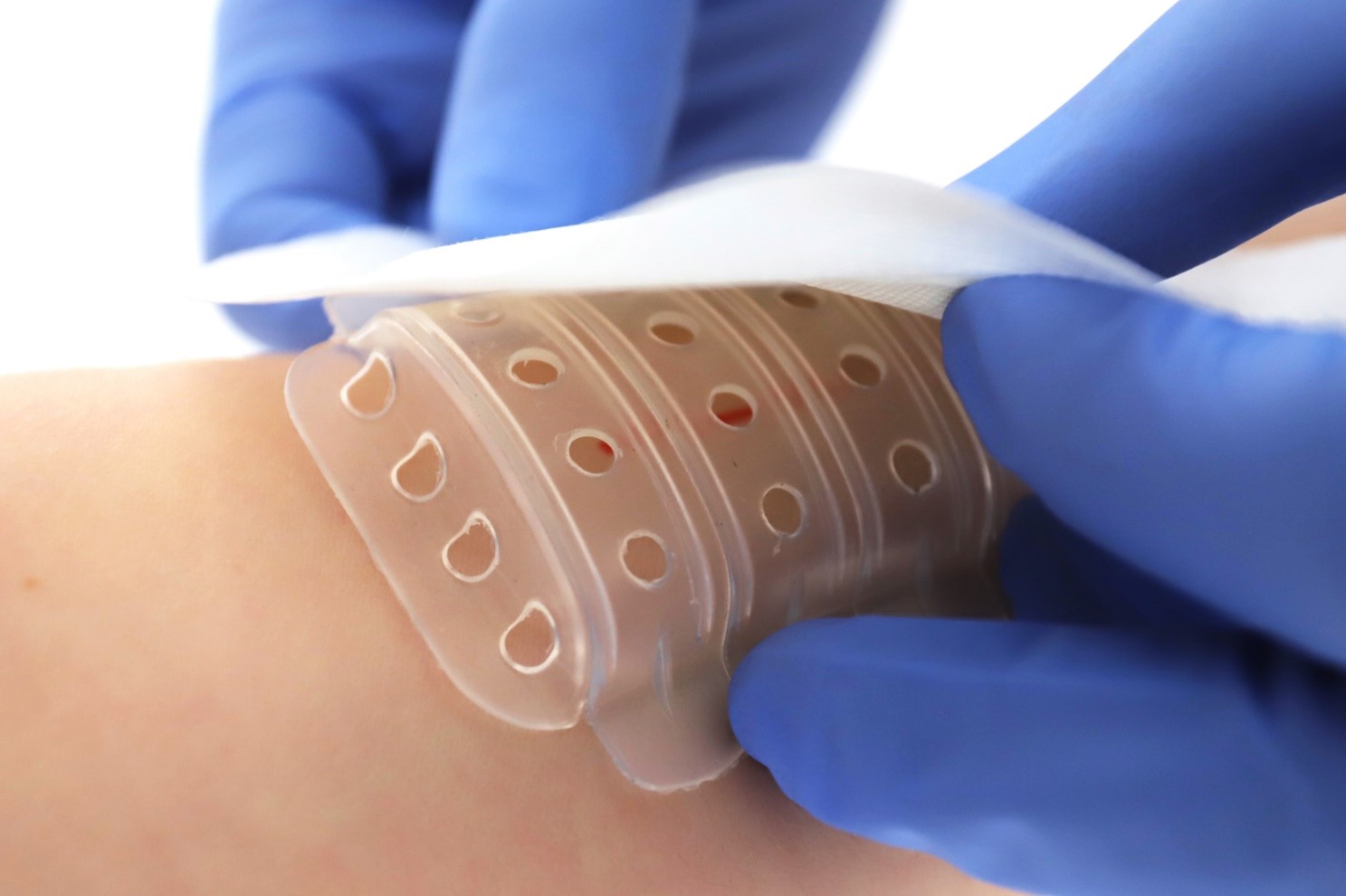
- An Italian company developed a groundbreaking medical dressing to optimize wound healing and patient comfort. This innovative wound spacer-shield ensures a sterile environment, promotes rapid healing, and reduces pain during dressing changes. The technology is now available for partnership opportunities to further develop and commercialize this solution. Potential partners could also contribute to clinical testing and regulatory approval processes.
- Full Description
-
An Italian company developed a new wound spacer-shield, made from hypoallergenic medical-grade TPE, offers a unique approach to wound care. The device is a pierced, cup-shaped shield designed to create a protective and sterile environment around the wound. This helps in promoting natural drying and rapid healing. The modular design allows for sections to be removed, fitting various wound sizes and types, including cuts, scrapes, surgical wounds, and burns.
Conventional dressings often stick to wounds, causing pain and disrupting the healing process. This spacer-shield eliminates such issues by preventing direct contact between the wound and the dressing. It allows for adequate ventilation, reduces the risk of infection, and facilitates easy access for applying creams and ointments.
The company is looking for partner to facilitate the commercialization of product.
Potential partners could also contribute to clinical testing and regulatory approval processes.
We are also open to potential investors in order to foster the development of StartUp. - Advantages and Innovations
-
Advantage of the innovation are:
- Sterile Protection: Maintains a sterile environment, reducing infection risk.
- Pain Reduction: Prevents dressings from adhering to wounds, minimizing pain.
- Modular Design: Adapts to different wound sizes and types.
- Enhanced Healing: Promotes natural drying and faster healing.
- User-Friendly: Easy application and maintenance. - Stage of Development
- Already on the market
- Sustainable Development Goals
- Goal 17: Partnerships to achieve the Goal
- Goal 3: Good Health and Well-being
- Goal 9: Industry, Innovation and Infrastructure
- IPR status
- IPR granted
Partner Sought
- Expected Role of a Partner
-
The ideal partner would be a company or institution with expertise in medical devices and wound care products. The partner is expected to facilitate its commercialization. Potential partners could also contribute to clinical testing and regulatory approval processes.
We are also open to potential investors in order to foster the development of StartUp. - Type and Size of Partner
- Big company
- SME 11-49
- SME 50 - 249
- SME <=10
- R&D Institution
- Type of partnership
- Investment agreement
- Commercial agreement with technical assistance
Dissemination
- Technology keywords
- 06001023 - Medical Furniture
- Market keywords
- 05004001 - Electromedical and medical equipment
- 05007007 - Other medical/health related (not elsewhere classified)
- 05004004 - Medical instruments
- Targeted countries
- All countries