Summary
- Profile Type
- Technology request
- POD Reference
- TRNL20250226022
- Term of Validity
- 27 February 2025 - 27 February 2026
- Company's Country
- Netherlands
- Type of partnership
- Commercial agreement with technical assistance
- Targeted Countries
- Germany
- Denmark
- Sweden
- Austria
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- A Dutch medTech development company is looking for medical manufacturing capacities, with cleanroom manufacturing possibilities and metal injection moulded small parts, for a cardovascular medical device. One manufacturer offering all facilities in house, or having several subcontractors in their network. Collaboration with a European partner extends the possibility to find the right partners that fit for their needs. Manufacturer is preferably located in Germany, Sweden, Austria or Denmark.
- Full Description
-
The Dutch Company is looking for collaboration with companies that have expertise in cardiovascular medical device manufacturing.
The product that the company is developing is based on the demands in the medical sector, by professionals and patients.
Cardiac surgeons are not able to perform patient-friendly minimally invasive coronary bypass procedures without the aid of coronary vessel connectors.The product the dutch company has developed makes cardiac surgery on patients more easy, less invasive and therefore patient friendly and lower on all cost. The minimally invasive procedure with a vessel connector significantly shortens hospital stay and recovery time, thereby reducing overall health care costs.
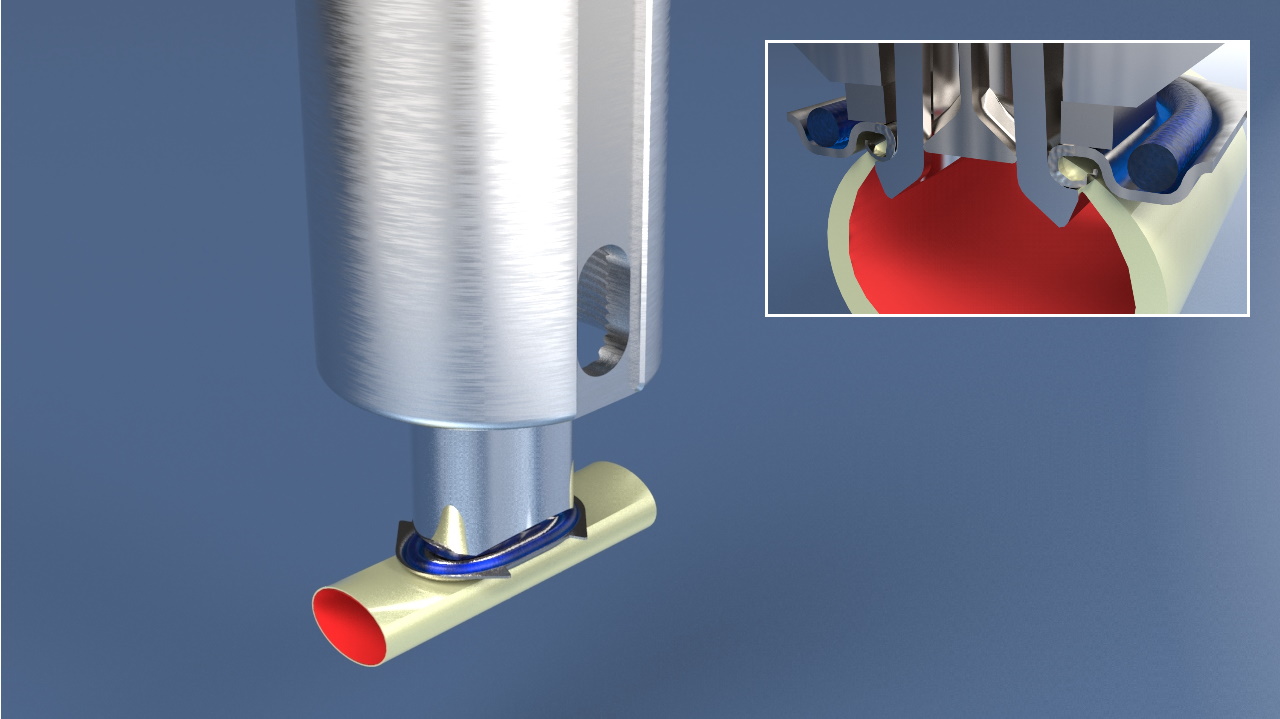
The product the company has developed to solve the needs is a Coronary Vessel Connector System, called the Octocon®. The Dutch company is currently on track with their development of this a coronary vessel connector to be marketed in Europe and US.
However, after the final stages of development, the product and parts need to be produced. Therefore the company is looking for a long term partnerschip with a foreign company (and their network of suppliers) to collaborate in the design for manufacturing of the product leading to a Contract cooperation with (a) Medical Manufacturing partner(s) abroad, in one of the mentioned countries Germany, Sweden, Austria or Denmark.
In the cooperation, the dutch company would need support in supplier selection, setting up the production line in a clean room environment.
The company expects from their cooperation partner(s) to have ISO-13485 certification, experience in the final stage of medical device development, including sterilization and packaging, clean-room for medical implant manufacturing, experience and know-how in Metal Injection Molding or 3D printing of miniature medical components with small tolerances and experience in Laser cutting of titanium for small medical components.
Based on the cooperation the company sees also opportunities in working with European SMEs to submit joined European subsidies and grants. - Advantages and Innovations
-
Surgeons will be able to perform coronary vessel connections in surgical situations where that is otherwise impossible due to technical challenges and space/approach restrictions. For patients this means a less complex surgery, with less invasive technology. This means also a much more comfortable and fast recovery of the surgery.
The innovative aspects of technology and product compared to current surgery methohds and technologies is the one-click mechanism to perform vessel connections in blood vessels of between 1.5 and 3.5 mm. However, this innovative technology is putting a burden on geometry, margins, and production methods. Therefore the dutch company is looking for cooperation with foreign companies who have expertise in the medical device manufacturing and production facilities in the mentioned levels. - Technical Specification or Expertise Sought
-
Clean room manufacturing for medical implant, with the following detailed expertise:
ISO-13485 certification, experience in the final stage of medical device development, including sterilization and packaging, clean-room for medical implant manufacturing, experience and know-how in Metal Injection Molding or 3D printing of miniature medical components with small tolerances, experience in Laser cutting of titanium for small medical components - Stage of Development
- Available for demonstration
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- IPR applied but not yet granted
- IPR notes
- not applicable
Partner Sought
- Expected Role of a Partner
- The dutch company is looking for collaboration with companies that have expertise in medical device manufacturing for this cardiovascular product. When starting the cooperation, the partner should be able to provide knowledge and support in design for manufacturing, setup manufacturing line, supplier selection and clean room manufacturing.
- Type and Size of Partner
- SME 11-49
- SME 50 - 249
- Type of partnership
- Commercial agreement with technical assistance
Dissemination
- Technology keywords
- 02002013 - Moulding, injection moulding, sintering
- 02002016 - Microengineering and nanoengineering
- 02002018 - Microassembly, nanoassembly
- 02002017 - Micromachining, nanomachining
- Market keywords
- 05004006 - Surgical instrumentation and equipment
- 05004004 - Medical instruments
- Sector Groups Involved
- Health
- Targeted countries
- Germany
- Denmark
- Sweden
- Austria