Summary
- Profile Type
- Technology offer
- POD Reference
- TODE20240206007
- Term of Validity
- 7 February 2024 - 6 February 2026
- Company's Country
- Germany
- Type of partnership
- Investment agreement
- Research and development cooperation agreement
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- A German university has developed a mechanism for powder inhalers that gives patients feedback on the effectiveness of their medication inhalation. The university offers a license and/or a technology cooperation agreement.
- Full Description
-
Capsule-based powder inhalers enjoy widespread use in the treatment of respiratory illnesses. Successful therapy depends not only on inhaler and active ingredient, but also on how patients use them. Inhalation at a low flow rate or for too short a time subjects the therapeutic effect to great fluctuation.
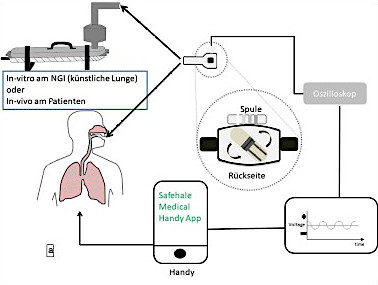
To give the patient feedback about inhalation effectiveness, scientists at a German university have developed a system from which conclusions can be drawn about inhalation quality and respirable particle quantity. The capsule rotation frequency during inhalation is used to determine airflow and inhalation duration. Rotation is read out by means of a neodymium magnet and a coil and can be used to determine such parameters as flow rate and emitted medication dose. In the future, a special app (currently in conceptual design) will enable the patient to receive the measured data easily and directly on his smartphone. He can use it to adjust his inhalation behavior, improving inhaled medication effectiveness.
The described setup is suitable for various commonly used and already established capsule-based powder inhalers. Various medication formulations can also be used and the system calibrated to them with little effort. The system does not depend on further parameters, specifically acoustic influences, so it can be used anywhere.
The system has been verified for various formulations and inhalers. A laboratory sample has been prepared and miniaturization planned. The university offers a technological cooperation agreement to medical technology companies and pharmaceutical industries in order to further develop this invention. A license agreement with the university is also desired. - Advantages and Innovations
-
The main advantages of this invention are:
- Quick feedback
- Small setup implementable in the inhaler
- Easy patient use
- Increased therapy effectiveness
- No interfering parameters - Stage of Development
- Lab tested
- Sustainable Development Goals
- Goal 9: Industry, Innovation and Infrastructure
- IPR status
- IPR applied but not yet granted
- IPR notes
- A patent has been applied for in Germany.
Partner Sought
- Expected Role of a Partner
- The university offers a technological cooperation agreement to medical technology companies and pharmaceutical industries in order to further develop this invention. A license agreement with the university is also desired.
- Type and Size of Partner
- SME 50 - 249
- SME 11-49
- Other
- Big company
- SME <=10
- Type of partnership
- Investment agreement
- Research and development cooperation agreement
Dissemination
- Technology keywords
- 09001005 - Mechanical Technology related to measurements
- 06001013 - Medical Technology / Biomedical Engineering
- 06005003 - Health information management
- 06005004 - Remote diagnostics
- Market keywords
- 03007002 - Other measuring devices
- 05005007 - Pulmonary medicine
- 05007004 - Monitoring equipment
- Sector Groups Involved
- Health
- Targeted countries
- All countries