Summary
- Profile Type
- Technology offer
- POD Reference
- TOFI20240523015
- Term of Validity
- 24 May 2024 - 24 May 2026
- Company's Country
- Finland
- Type of partnership
- Commercial agreement with technical assistance
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
-
The family owned company has over 20 years of experience in laser-marking and CSSD solutions. They manufacture:
1) laser-marked multi-colored lables for surgical intruments and accessories
2) a custom-made instrument tracking software.
These innovative products can be used together or separately.
They are looking for CSSD contacts in hospitals as well as medical device distributors especially in Germany and Belgium. - Full Description
-
The tracking labels have been in use in Finnish CSSD facilities since 2021, making it a pioneering innovation.
The labels are suitable for most materials commonly found on instruments, guaranteeing compatibility. Whether scanned by machine vision systemes of visually inspected by human eyes, the labels offer clear readabilty ensuring accurate identification and tracking. Built to withstand the demanding conditions of CSSD operations, the labels are engineered for long-lasting durability throughout the lifecycle of instruments while still being removable, without leaving behind residue or damage.
Manufactured to order, the own set of requirements and preferences of every CSSD department is taken into account. The company offers fully customizable tracking labels in a variety of colors. The most common and recognized sizes are round with diameters 4.5 mm, 5.0 mm and 6.5 mm. Rectangular labels are also available, for long and narrow instruments. Upon request, 2D matrix code (ECC200) can be laser-marked on each lable. All matrix codes meet GS1 standards. Each color has clearly contrasting markings for superior machine vision and human eye readability.
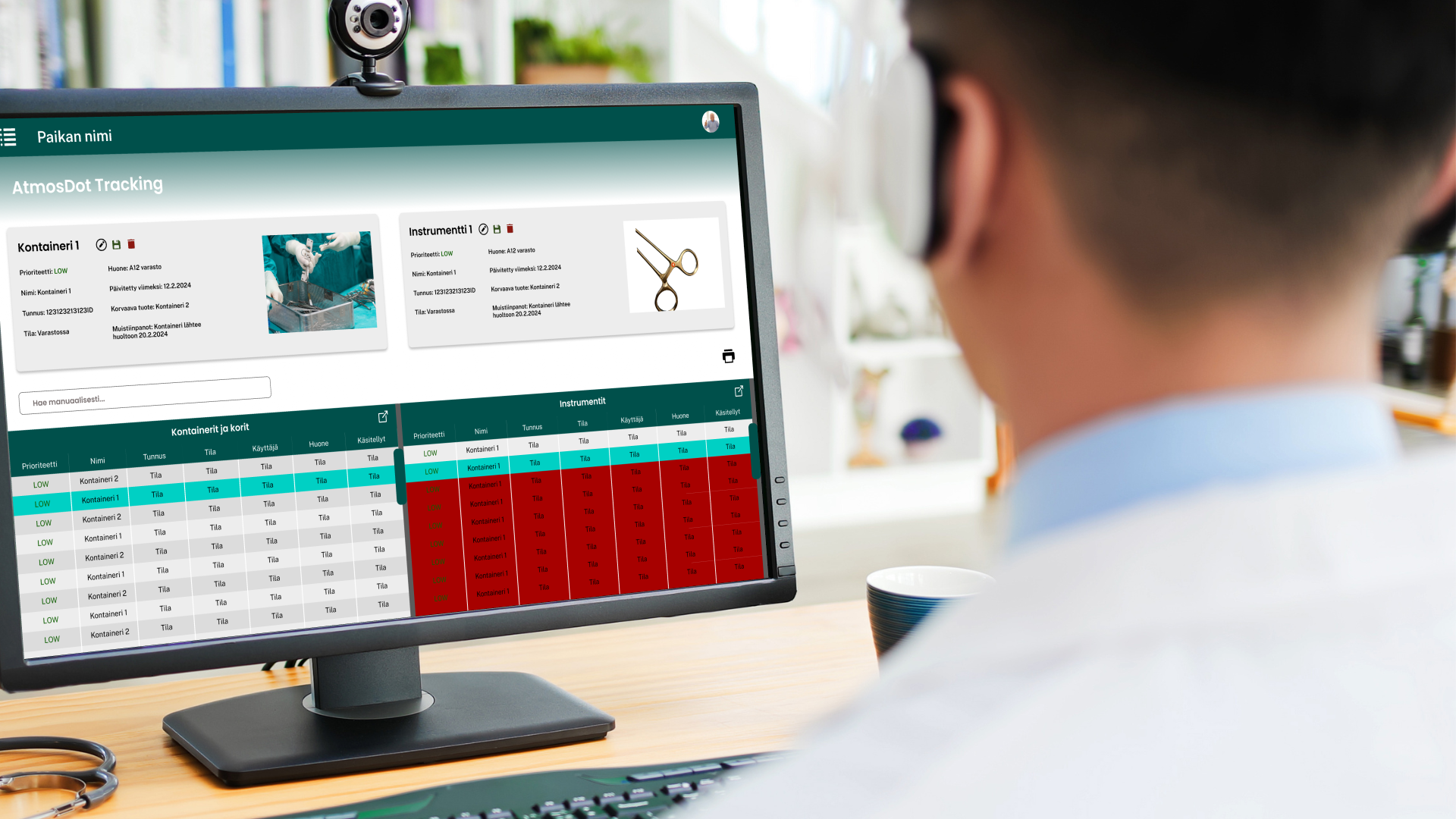
With seamless integration between the tracking labels and custom software, the instrument tracking process can be streamlined and workflow optimized while minimizing manual data entry. Boosting efficiency and accuracy and extending surgical instruments' lifespan, the software offers CSSD management precise, real-time data for smarter decision-making.
Key features are individualised instrument identification, detailed sterilization cycle monitoring, tailored alerts and notifications, seamless integration and user authentication and enhanced user experienc and scalability. The company offers automatic updateds and continuous support.
Color-coded labels can be used alone or integrated with the tracking software.
The labels have been widely embraced in Finland for their vibrant colors and effectiveness in managing instruments and accessories. They've become essential in CSSD-maintenance operations, serving almost the whole country.
The company has been operating solely in the domestic market and is now eager to explore international trade opportunities.
The products are truly unique, and the two-decade track record as a leading solution provider in laser marking in the Finnish market emboldens the company to expand into new markets, confident in the value they bring.
Lastly, the company has established a collaboration with an official CSSD institution. Developing the CSSD- EPR system together with actual users, educational institutions, and hospitals ensures the system is user-friendly, efficient, and meets the needs of the staff. - Advantages and Innovations
-
The only fully customizable color coded labels and tracking software solution for surgical instruments in the market.
The solution offers increased operational efficiency, improved compliance with health and safety regulations, data-driven insights for continuous process improvement, reduction of overload, maintenance and repair scheduling, streamlined workflow and enhanced communication between operating rooms and sterile processing in hospitals. - Technical Specification or Expertise Sought
- Central sterile services departments in hospitals and medical device distrubutors.
- Stage of Development
- Already on the market
- Sustainable Development Goals
- Goal 8: Decent Work and Economic Growth
- Goal 3: Good Health and Well-being
- Goal 4: Quality Education
- Goal 9: Industry, Innovation and Infrastructure
- IPR status
- IPR granted
- IPR notes
- IPR granted for labels
Partner Sought
- Expected Role of a Partner
- Central sterile services departments in hospitals and medical device distrubutors.
- Type and Size of Partner
- Big company
- Other
- SME 50 - 249
- SME 11-49
- Type of partnership
- Commercial agreement with technical assistance
Dissemination
- Technology keywords
- 06001013 - Medical Technology / Biomedical Engineering
- 01004012 - Operation Planning and Scheduler System
- 01004001 - Applications for Health
- Market keywords
- 05004004 - Medical instruments
- 05004006 - Surgical instrumentation and equipment
- 02006009 - Other computer services
- 02007001 - Systems software
- 05007007 - Other medical/health related (not elsewhere classified)
- Sector Groups Involved
- Health
- Targeted countries
- All countries