Summary
- Profile Type
- Technology offer
- POD Reference
- TOGR20250624004
- Term of Validity
- 24 June 2025 - 24 June 2026
- Company's Country
- Greece
- Type of partnership
- Commercial agreement with technical assistance
- Investment agreement
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- A Greek medtech company has developed a system, the first over-the-counter (OTC) molecular diagnostic test for sexually transmitted infections (STIs), offering lab-level accuracy at home in under 20 minutes. The company is seeking strategic partners, such as pharmaceutical, diagnostic or medtech companies, to support regulatory certification and large-scale commercialization under investment, joint venture or licensing agreements.
- Full Description
-
The Greek company is a biotechnology firm specializing in the development of advanced molecular diagnostic solutions. It brings together expertise in biomedical engineering, microfluidics, molecular biology, and software integration to design high-performance, accessible diagnostic technologies. Backed by a multidisciplinary team and strong academic and industrial partnerships, the company is committed to delivering innovative platforms that support decentralized, reliable, and timely diagnostics.
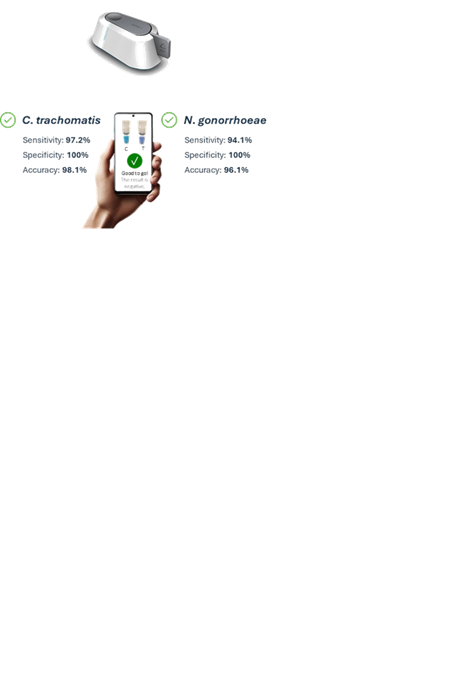
In line with this mission, the company has developed a system—the world’s smallest molecular diagnostic device for sexually transmitted infections (STIs). This pocket-sized, at-home testing tool enables users to detect Chlamydia trachomatis and Neisseria gonorrhoeae with clinical-grade accuracy in less than 20 minutes. Designed for ease, speed, and discretion, this system empowers individuals to take proactive control of their sexual health through private, fast, and reliable screening.
Unlike conventional lateral flow antigen tests—which often fail to detect low viral loads—the company’s solution leverages advanced molecular diagnostics to identify even asymptomatic infections. This approach significantly enhances early detection and limits the silent transmission of STIs, especially in individuals who do not exhibit symptoms. The solution does not replace professional diagnosis but acts as a reliable first line of detection, reducing testing barriers and alleviating strain on healthcare systems. By encouraging regular self-screening, this system supports early treatment, reduces transmission rates, and enhances public health.
The technology is progressing through final development milestones. The system’s hardware device has achieved TRL 8, while the associated test chemistry is at TRL 6. The path to TRL 9 includes CE marking for in vitro diagnostic (IVD) devices (CE-IVD) regulatory certification, clinical sample validation, pilot batch production, and real-time stability studies. In parallel, the company is preparing for FDA clearance through the De-Novo or 510(k) pathway, involving extensive clinical trials with over 4,500 participants.
To accelerate time to market and maximize societal impact, the company is seeking
strategic partners to support regulatory certification (CE-IVD, FDA) and enable large-scale commercial deployment. Ideal partners include pharmaceutical, diagnostic, and medtech companies with strong market presence, regulatory expertise, or distribution networks. Collaboration may take the form of an investment agreement, commercial agreement with technical assistance, joint venture, or licensing arrangement, offering shared value and market growth potential. - Advantages and Innovations
-
The global STI diagnostic market is expected to grow from EUR 9.25 billion in 2022 to EUR 18.29 billion by 2032. Demand is shifting from less sensitive lateral flow tests to more accurate molecular diagnostics, which can detect even asymptomatic infections. The competitive landscape includes Point-of-Care (POC) kits from major players. These are CE/FDA-cleared and used in clinical environments, offering advanced diagnostics and single-visit treatment. However, their reliance on healthcare settings and professionals limits accessibility for those seeking privacy or testing outside medical facilities.
The Greek company’s device disrupts this landscape as the first full over-the-counter (OTC) molecular diagnostic STI test. It delivers accurate results in under 20 minutes, directly at home, offering unmatched autonomy, discretion, and speed. This innovation aligns with post-Covid consumer expectations for rapid self-testing and aims to significantly reduce STI transmission through early detection.
Targeting adults aged 18–39—particularly the 69% of 18–29-year-olds who identify as single—this system is positioned for high uptake among health-conscious, sexually active individuals. With an addressable market of over 50 million adults, the company projects demand for 10 million tests annually, representing a potential revenue opportunity exceeding €1 billion. - Stage of Development
- Available for demonstration
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- IPR applied but not yet granted
- IPR notes
- More information upon request.
Partner Sought
- Expected Role of a Partner
-
The Greek company has advanced their innovative device to TRL 8 and the associated STI test chemistry to TRL 6. To unlock its full market potential and deliver measurable public health impact, the company needs strategic partnerships to finalize its regulatory certification and transition to large-scale commercial deployment. Achieving CE-IVD and FDA approvals and scaling production to commercial volumes requires a strategic collaboration with an established pharmaceutical, diagnostic, or medtech partner.
The ideal partner would provide complementary resources—financial investment, regulatory expertise, technical capacity, or distribution infrastructure—to accelerate the certification process and support market introduction. The collaboration may take the form of either an investment agreement or a commercial agreement with technical assistance, depending on the partner's strengths and strategic interests. Specifically, this could include an investment agreement (e.g., equity participation or milestone-based funding), a joint venture (establishing a shared legal entity for development and commercialization), or a license agreement (granting commercialization rights in defined territories in exchange for royalties, upfront payments, or revenue share). Each of these models supports a balanced approach to risk-sharing and long-term value creation.
As the first OTC molecular test for STIs, this system offers lab-grade accuracy in a compact, private, and easy-to-use format. With the STI diagnostic market expected to surpass €18 billion by 2032, the company’s solution is well positioned to meet the rising demand for discreet and accessible testing. A strong commercial partnership will be key to accelerating its market entry and expanding its global public health impact. - Type and Size of Partner
- Big company
- SME 50 - 249
- Type of partnership
- Commercial agreement with technical assistance
- Investment agreement
Dissemination
- Technology keywords
- 06001005 - Diagnostics, Diagnosis
- 06002002 - Cellular and Molecular Biology
- 06001015 - Pharmaceutical Products / Drugs
- 06002009 - Molecular design
- Market keywords
- 05004005 - Diagnostic equipment
- 05001005 - Molecular diagnosis
- 05001001 - Diagnostic services
- 04011 - Molecular design
- Sector Groups Involved
- Health
- Targeted countries
- All countries