Summary
- Profile Type
- Technology offer
- POD Reference
- TOGR20250609009
- Term of Validity
- 9 June 2025 - 9 June 2026
- Company's Country
- Greece
- Type of partnership
- Investment agreement
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- A novel and globally patented combination of drugs displays a unique cytolytic action against malignant cells in the brain. A Greek Hospital seeks a Pharmaceutical partner to further its clinical trials and to facilitate regulatory approval through an investment agreement and/or a research cooperation agreement.
- Full Description
-
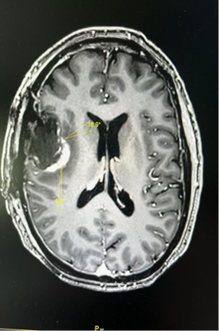
Individuals diagnosed with brain tumors of high malignancy (high grade gliomas) generally have a prognosis of less than one year. However, the proposed novel drug has demonstrated remarkable success in the first two patients, who are alive and well 7 and 2 years respectively after treatment, with no evidence of recurrence or any clinically significant side effects. Notably the second patient exhibited a unique MRI proof of a glioblastoma destruction within five days due to the drug's action - see images below.
As high grade gliomas are considered a rare condition with a dismal prognosis and no effective treatment, it allows for the novel drug to be designated an "orphan drug”. A pharmaceutical company with such a novel drug can therefore benefit from significant marketing advantages globally.
The Greek Company’s goal is to partner with a pharmaceutical company adept at navigating the regulatory frameworks within the EU and US, so as to establish an officially registered clinical study of the above treatment, obtain “orphan drug designation” for the novel drug and prove its clinical superiority over existing therapies for high grade gliomas. This is envisioned through a research cooperation agreement. Investors are also sought to help fund the clinical trials through an investment agreement. - Advantages and Innovations
-
• No drug resistance development due to unique cytolytic action
• Continuously sufficient drug concentration at target cells, for effective therapy, delivered through intratumorally indwelling catheters: not possible with systemic drug administration
• Phased administration of the drug in alkaline solutions and aspiration of pathological extracellular fluid directly impacts the Warburg effect and evacuates extracellular vesicles, respectively, thus erasing key contributors to the cancer’s sustenance and progression.
• Negligible side effects compared to standard therapies
• Patent granted in the UK and US; worldwide PCT (Patent Cooperation Treaty) application ongoing.
• The manually operated indwelling catheter system consists of “off the shelf” routine surgical equipment. - Stage of Development
- Under development
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- IPR granted
Partner Sought
- Expected Role of a Partner
-
In the context of a research cooperation agreement, or possibly an investment agreement, a pharmaceutical company is sought to:
1) assume the creation of a new drug file for pre-clinical trials
2) collaborate with clinical research organizations for officially registered clinical trials in EU, India and USA
3) obtain orphan drug designation
4) explore the drug’s applications to malignant tumors outside the Central Nervous System
5) contribute to the development of a novel, patented drug delivery system for invasive intratumoral chemotherapy
The ideal collaborator should be an established drug manufacturer, with a proven track record in obtaining regulatory approvals for novel drugs by EMA (European Medicines Agency) and FDA (Food and Drug Administration, USA) and expertise in the regulatory process for orphan drugs. - Type and Size of Partner
- Big company
- Other
- Type of partnership
- Investment agreement
Dissemination
- Technology keywords
- 06001003 - Cytology, Cancerology, Oncology
- 06001002 - Clinical Research, Trials
- 06001012 - Medical Research
- Market keywords
- 05005014 - Oncology
- 05005022 - Other clinical medicine
- Sector Groups Involved
- Health
- Targeted countries
- All countries