Summary
- Profile Type
- Technology offer
- POD Reference
- TOKR20241028003
- Term of Validity
- 29 October 2024 - 29 October 2025
- Company's Country
- South Korea
- Type of partnership
- Commercial agreement with technical assistance
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- A Korean biotech SME has developed a tiny nuclease augment RNA-based genome editing technology. Compared to existing CRISPR-Cas9, the developed technology provides low off-target effect and immunogenicity and high deliverability because it is based on a hypercompact Cas nuclease. This company is seeking partners interested in using this gene editing technology under a license agreement.
- Full Description
-
Genome editing, also called gene editing, allows genetic material to be added, removed, or altered at a particular locations in the genome. This powerful tool is generally used for gene function researches, the development of high value genetically modified animals and plants, and the treatment of genetic diseases and intractable diseases.
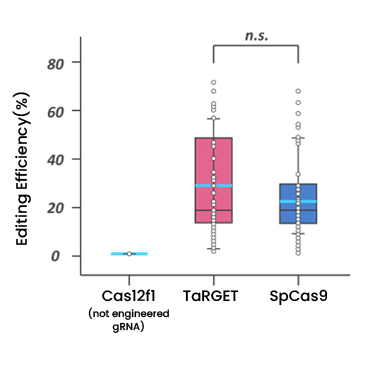
A Well-known and widely-used one is called CRISPR(Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 (associated protein 9). Even though this CRISPR-Cas9 is faster, cheaper, more accurate, and more efficient than previous gene editing technologies, it has limitations such as large size (4.1kb) and high frequency of off-target effects. (≥ 50%)
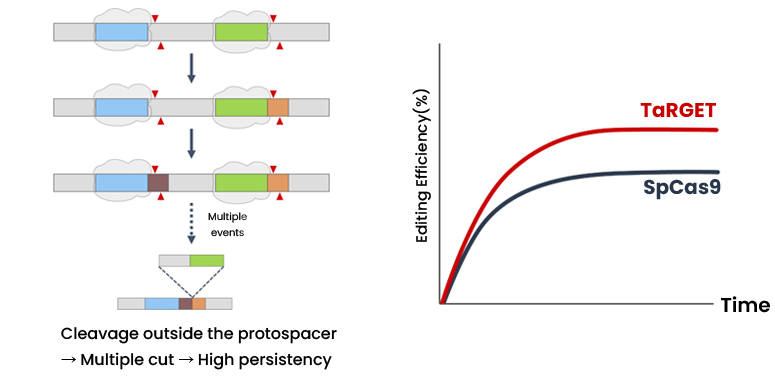
A Korean SME have developed CRISPR-Cas12f platform technology with a mini-nuclease and proprietary gRNA, so called TaRGET (Tiny Nuclease Augment RNA-based Genome Editing Technology) system. With this platform technology, researchers can disrupt, delete, gene-regulate, substitute, correction and insertion genomes as needed. Compared to existing CRISPR-Cas9 technology, this new technology is based on hypercompact Case nuclease and provides many advantages such as low off-target activity and lower immunogenicity concern. Additionally, due to its smaller size (about 40%) than Cas9, it can be delivered using a single AAV(adeno-associated viruse) for in vivo therapy. Utilizing these excellent advantages, the Korean company is researching gene editing therapy for ocular, muscular, CNS (Central Nervous System), and liver diseases.
Regarding its intellectual property of technology, the company has two IPs; engineered gRNAs and engineered Cas12f1 to increase gene editing efficiency. These IPs have been applied for and granted in major countries including the US, EU, Japan, China, and South Korea. With its technological excellence and protected IPs, the Korean company has signed several R&D collaboration and license agreements to develop in vivo gene therapy with foreign biotech companies in the USA.
This company is seeking Euroepan partners such as CDMO (Contract Development and Manufacturing) or CRO (Contract Research Organization), who are interested in this gene editing technology for their researches or development of new drugs under a license agreement. - Advantages and Innovations
-
Compared to CRISPR-Cas9 technology, the Korean company’s technology has various advantages such as,
- Size: 1.7 kb (1/3 of Cas9)
- Deliverability: delivered using a single AAV (Adeno-associated viruses) for in vivo therapy
- Low level of off-target activity
- Lower immunogenicity
In addition, this technology is free from intellectual property (IP) issues related to Cas9 because the company has its own IP. - Stage of Development
- Already on the market
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- IPR granted
Partner Sought
- Expected Role of a Partner
- CDMO (Contract Development and Manufacturing) or CRO (Contract Research Organization), who are interested in license-in Korean SME’s gene editing technology for their internal and/or external purpose under a license agreement.
- Type and Size of Partner
- R&D Institution
- Big company
- SME 11-49
- SME 50 - 249
- Type of partnership
- Commercial agreement with technical assistance
Dissemination
- Technology keywords
- 06002002 - Cellular and Molecular Biology
- 06003001 - Bioinformatics
- 06001009 - Gene - DNA Therapy
- 06002005 - Genetic Engineering
- Market keywords
- 04008 - Genetic Engineering
- 05003001 - Therapeutic services
- Sector Groups Involved
- Health
- Targeted countries
- All countries