Summary
- Profile Type
- Technology offer
- POD Reference
- TOIT20240923013
- Term of Validity
- 3 October 2024 - 3 October 2025
- Company's Country
- Italy
- Type of partnership
- Commercial agreement with technical assistance
- Research and development cooperation agreement
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
- An Italian CDMO specializing in first-in-class biotherapeutic development and manufacturing of biologics provides high-quality, services for biopharmaceutical companies from cell line development to GMP manufacturing of drug candidates. The company has built platforms for lead candidate selection, optimization of cell lines, process development and optimization as well as drug substance and drug product manufacture from early clinical to commercial phase. Commercial partnerships are sought.
- Full Description
-
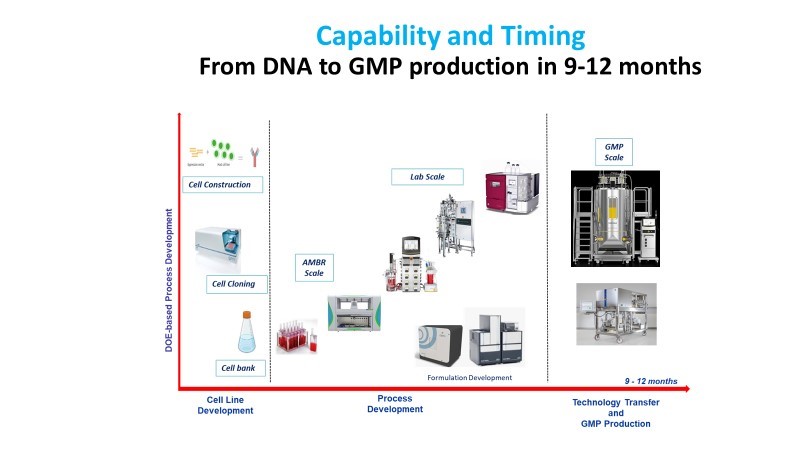
The process development is a critical step for the clinical product development in the field of biologics, so much so that even today “the product is the process” and the optimization of manufacturing processes has to ensure the efficient and scalable production of biologics.
Built-in Quality by Design (QbD) in process development reduces process variability yielding robust reproducible processes to be scaled up to the final manufacturing scale with a reduced timeline. The company offers services for both Upstream and Downstream process development of biotechnological products focusing on increasing efficiency and reducing development timelines using the state of art HighThroughput technologies available. The processes are tailored on both product specifications and client needs, selecting the best applicable solutions for biologics processing and by applying QbD approaches in any stage of the process development.
Consolidated purification platforms for monoclonal antibodies and bispecific antibodies, which allow shortening timelines and effort to transfer the process in manufacturing, are also available.
Hands on experience in performing scale down models on laboratory scale validating the process and ensure the scalability complete the offer. The main workflow includes:
1. Process Design
a) Upstream Process Development:
i. Media optimization and scale-up of cell culture processes up to 50L single use bioreactors.
ii. Optimization of cultivation conditions (e.g.temperature, pH, DO)
iii. Primary recovery optimization
iv. Harvest product characterization (incl.viral safety) and stability studies
b) Downstream Process Development:
i. Development of purification methods (chromatography, filtration).
ii. Optimization of recovery steps (centrifugation, precipitation).
iii. Formulation development for stability and delivery
iv. Hold points and purified bulk product stability studies
c) Bioconjugation (if applicable)
i. Payload and linker synthesis development
ii. Payload-linker synthesis optimization
iii. ADC optimize conjugation w/wout purification protocol
iv. Formulation development for stability and delivery
v. ADC stability studies
2. Process Scale-Up and Technology Transfer
a) Conduct pilot-scale studies to demonstrate process scalability.
b) Set up appropriate Control Strategy
c) Transfer the developed process to manufacturing facilities (internal or third-party) while ensuring all necessary documentation and training.
3. Process Validation&Characterization
a) Prepare Validation Process Master Plan, Process Risk Assessment and any other document required to validate the entire process to ensure consistency, reliability, and product quality.
b) Conduct process performance qualification (PPQ) runs to demonstrate that the process performs as intended.
c) Continous Process Verification
d) Characterisation End of Production Cell Bank and in vitro cell age
e) Scale down models for Upstream Process Characterization Studies
i. Small scale media expiry studies
ii. Small scale model qualification
iii. Small scale pre-characterisation
iv. Small scale process characterisation
v. Worst Case runs
vi. Cell line stability
d) Scale down models for Viral Clearance and Downstream Process Characterization Studies
i. Intermediate stability & hold time validation
ii. Buffer expiry and mixing validation
iii. Impurity spiking studies
iv. Resin & Membrane storage studies-Bench scale
v. Resin & Membrane cleaning studies-Bench scale
vi. Leachable and Extractable Risk assessment and/or studies
vii. Cleaning Validation
4. Regulatory Submission Support
a) Final Report: Provide comprehensive documentation detailing methods and results
b) Regulatory Support: Assist with documentation necessary for regulatory submissions (e.g., IND applications) as required by the client. - Advantages and Innovations
-
The following points represnets a strong opportunity and innovative approaches:
1) Service is offered by a Long-term vision and financially stable company
2) Quick and reaction and short lead times to start project
3) Functional Service Provider model applied, to offer more flexibility and by allowing start-up/small biotech companies to engage us for specific tasks or work packages, such as process development starting from scratch or tech transfer of lab scale process and development for clinical phase applications.
4) Advantage from many years of experience in applying ICHQ8, ICHQ9 and ICHQ10 regulatory guidelines permits the aligning of the development of the process of your product with the latest regulatory expectations.
6) Accelerate your program by relying on fast and accurate workflows
7) Gain confidence and enhance the knowledge of your product through a robust validation framework - Stage of Development
- Already on the market
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- Secret know-how
Partner Sought
- Expected Role of a Partner
-
Expected role of the partner
The partners sought for are:
• Public and/or private entities
• Pharmaceutical companies for testing lines of their products
• Research centres and universities for partnering in research projects - Type and Size of Partner
- R&D Institution
- SME 50 - 249
- SME 11-49
- Type of partnership
- Commercial agreement with technical assistance
- Research and development cooperation agreement
Dissemination
- Technology keywords
- 06001002 - Clinical Research, Trials
- 06001012 - Medical Research
- 06001015 - Pharmaceutical Products / Drugs
- 06006012 - Bioprocesses
- Market keywords
- 05007002 - Pharmaceuticals/fine chemicals
- 05007007 - Other medical/health related (not elsewhere classified)
- 05007005 - Hospital and other institutional management
- Sector Groups Involved
- Aerospace and Defence
- Health
- Targeted countries
- All countries