Summary
- Profile Type
- Technology offer
- POD Reference
- TOES20250708003
- Term of Validity
- 8 July 2025 - 8 July 2026
- Company's Country
- Spain
- Type of partnership
- Commercial agreement with technical assistance
- Targeted Countries
- All countries
Contact the EEN partner nearest to you for more information.
Find my local partner
General information
- Short Summary
-
A Spanish healthcare research institute has developed a simulator that allows the training of specialists in any technique of respiratory endoscopy, through realistic simulation. The simulation device overcomes the drawbacks of training over artificial organs and live or dead bodies.
They would like to talk to companies interested in the fabrication and distribution of this device under a commercial agreement with technical assistance. - Full Description
-
The Spanish healthcare institute is specialized in promoting and developing research and innovation in the bio-health environment, with the aim of seeking solutions to health problems and contributing to scientific, educational, social and economic development.
Synthetic cardiopulmonary block simulators do not allow for the training of certain endoscopic techniques. On the other hand, the use of cadavers or live animals is costly. The simulation device for respiratory endoscopy techniques presented here allows for the practice of endoscopies on a cardiopulmonary block of animal origin without the need for the animal's body. It allows the use of actual organic tissue without the need to use live or deceased patients or animals.
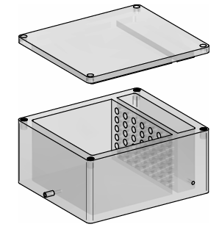
The simulator consists of a box, a drainage tube, a tube, and a removable upper lid. In the working position, the box, formed by a bottom and side walls, comprises a working chamber and a suction chamber, both separated by a breathable intermediate membrane. The tube passes through a wall of the working chamber where the cardiopulmonary block (trachea and lungs tissue) is fixed, and the drainage tube passes through a wall of the suction chamber where air is sucked from both chambers to a negative pressure.
During use, the working chamber houses the cardiopulmonary block fixed in the tube at the second end, and the suction chamber has the drainage tube to aspirate the air. Since the intermediate membrane that separates both chambers is breathable, when suction is performed in the suction chamber, suction is also created in the working chamber. As this low pressure is generated by the suction in the chambers, the cardiopulmonary block expands to occupy the space of the working chamber, which allows for endoscopy practices by introducing the endoscopic element into the expanded cardiopulmonary block through the tube.
This simulator is useful in hospital units and training and simulation centres, such as virtual hospitals, for the training of pulmonology residents and other related professionals in the management of the cardiopulmonary block and the airways.
The research institute would like to talk to companies interested in the fabrication and distribution of the simulator to cooperate in the framework of a commercial agreement with technical assistance. - Advantages and Innovations
-
The main competitive advantages of the device are:
- Simple.
- Realistic. Avoiding in vivo experimentation.
- Versatile. Allows training in any endoscopic technique, in addition to simulating tumors or lesions.
- Flexible. Enables quick and easy change of the cardiopulmonary block.
- Cost-effective. - Technical Specification or Expertise Sought
- Health care related firms
- Stage of Development
- Available for demonstration
- Sustainable Development Goals
- Goal 3: Good Health and Well-being
- IPR status
- IPR granted
- IPR notes
- A utility model covering this technology has been granted by the Spanish Patent Office with reference ES1312494U.
Partner Sought
- Expected Role of a Partner
- Commercialization of health care simulation devices, manufactures, distributors.
- Type and Size of Partner
- Big company
- SME 50 - 249
- SME 11-49
- SME <=10
- Type of partnership
- Commercial agreement with technical assistance
Dissemination
- Technology keywords
- 06001013 - Medical Technology / Biomedical Engineering
- 09001002 - Analyses / Test Facilities and Methods
- 06001002 - Clinical Research, Trials
- 07001009 - Veterinary Medicine
- Market keywords
- 05007007 - Other medical/health related (not elsewhere classified)
- 05005007 - Pulmonary medicine
- 05004004 - Medical instruments
- Sector Groups Involved
- Health
- Targeted countries
- All countries